Submission of an Article
To reduce delays, authors should adhere to the level, length, and format of the journal at every stage of processing right from manuscript submission to each review stage. Editable Word files are required to typeset your article for final publication. All correspondence, including notification of the Editor's decision and requests for revision, is sent by e-mail.
Contact Information
For queries with your submission, please contact the team via email:
Editor in Chief
Dr. Marcello Iriti
Email:
Contribution of Author
The credit of the authorship should be based on
Consequential inputs to the concept and design, procurement of data or analysis, and interpretation of data.
Preparing the article or revising it critically for an important matter.
Final confirmation of the adaptation to be published. All three conditions must be met by the authors. Participation solely in the acquisition of funding or data or general supervision of the research group does not constitute authorship.
The author must include a proclamation mentioning each author's contribution. Please ensure that this is discussed with your co-authors and that compliance is reached before manuscript submission. Post-acceptance changes to the author list will not be permitted. The contribution statement is not included in the maximum word count.
Article Types
Letter to the Editor
A Letter to the Editor is a brief report that is within the journal's scope and of particular interest to the community, but not suitable as a standard research article. It does not follow a format such as an abstract, subheads, or acknowledgments. It is more feedback or the opinion of the reader on a particular article published and should reach the editor within 6 months of article publication. Letters should have a maximum of 1,500 words.
Original Articles
The Research Articles report on primary research. They must describe significant and original observations. Consideration for publication is based on the article’s originality, novelty, scientific soundness, and appropriateness of its analysis. Research articles should follow all the criteria mentioned in the preparation of the manuscript section and have a maximum of 5,000 words (Excluding references), a maximum of 50 references (70% of the references should be within the last 5 years), and 7 tables/figures together. Please use the journals-ready template for preparing your article.
Review Articles
Review Articles are considered reviews of research or summary articles. They are state-of-the-art papers covering a current topic by experts in the field. They should give evidence and provide answers to a well-defined aspect or question in a particular area. The introduction generally delivers the issue forward to the readers followed by analytical discussion with the help of imperative tables, graphs, pictures, and illustrations wherever necessary. It compiles the topic with a conclusion. All the statements or observations in the review articles must be based on necessary citations, providing complete references at the end of the article. Review articles should have a maximum of 7,000 words (Excluding references), and a maximum of 100 references (70% of the references should be within the last 5 years). Please use the journals-ready template for preparing your review article. Abstract, keywords, introduction, and conclusion headers are necessary.
Case study
A case study is a detailed examination of a single instance, event, or subject, often used to explore and highlight unique clinical, organizational, or situational phenomena. This type of study provides an in-depth understanding of complex issues, offering valuable insights into the practical application of theories and practices. Case studies are particularly effective in shedding light on rare or novel occurrences that may not yet be widely documented. Submissions in this category should follow a structured approach, including an introduction, a detailed presentation of the case, a discussion of its implications, and conclusions that link the findings to broader contexts. The word limit for case study manuscripts is 3,000 words, excluding references and tables, and authors are encouraged to include up to 50 references to support their findings and analysis.
Cross-Sectional Study
A cross-sectional study is a type of observational research that analyzes data from a population, or a representative subset, at a single point in time. These studies are particularly useful for determining prevalence rates, identifying associations between variables, and generating hypotheses for further longitudinal research. Cross-sectional studies often involve surveys, medical records, or other forms of data collection, providing a snapshot of the studied phenomenon. Manuscripts submitted in this category should clearly describe the study design, population, sampling methods, and statistical analyses used. Like case studies, cross-sectional study submissions are limited to 3,000 words, with up to 50 references to ensure thorough and credible reporting of findings. Authors should emphasize the practical implications of their results while discussing their study's strengths and limitations.
Clinical trial
All Clinical Trials should be registered in a publicly available registry approved by the WHO or other organizations and the clinical trial number must be clearly stated in the manuscript.
Plagiarism
At any stage of peer review, publication, or post-publication, if plagiarism is detected the manuscript may be rejected, returned to the author for correction, or retracted.
Peer Review
We use a double-blind peer-review system where both the referee and author remain anonymous throughout the process. We aim to provide authors with timely and constructive feedback regarding their submitted manuscripts.
Authors’ Professional and Ethical Responsibilities
BPRMCS follows the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Works in Medical Journals (Available at: https://icmje.org).
Authorship
Only those persons who contributed directly to the intellectual content of the article should be listed as authors. The surnames and addresses/affiliations of all authors, as well as the e-mail address of the corresponding author, should be indicated on the title page. Only one person can be the “Corresponding Author”. The corresponding author is the one individual who takes primary responsibility for communication with the journal office during the manuscript’s submission, peer
review, and publication processes.
Acknowledgments and Sources of Support
Sources of outside support for the research work, including funding, grants, equipment, and drugs, must be indicated in the Acknowledgements section. Any involvement of laboratory support staff and other researchers must also be clearly stated in the Acknowledgement statements.
Double Publication and Concurrent Submission
Duplicate or Double submission means submission of a manuscript to two or more different journals at the same time. Manuscripts that are submitted to the BPRMCS should not have been previously published in, submitted to, or be under consideration for publication by another journal.
Any manuscript displaying more than 15% level of duplication by the journal’s iThenticate/Plagiarism check will not be considered for publication in the journal.
Ethical Considerations
All manuscripts submitted to the BPRMCS must provide appropriate information on Ethical Considerations. Research works on laboratory animals must provide evidence of institutional or national ethical approval.
When reporting experiments on animals, authors should indicate whether the institutional and/or national guidelines for the care and use of laboratory animals were followed. Voucher numbers of plant materials used in the study must also be provided.
All studies involving human subjects must expressly include written consent of the patients or volunteers. If the patient is deceased or incapable of providing informed consent, the patient’s next-of-kin, beneficiary, or legal guardian should provide the consent. Inclusion and Exclusion criteria for the choice of patients or volunteers used must also be clearly stated. When reporting experiments on human subjects, authors should indicate whether or not the procedures followed are in agreement with the ethical standards of the committee on human experimentation (institutional, national, and international), and with the Helsinki Declaration of 1975, as revised in 2008.
Preparation of Manuscript
Title
The first page should contain a short title (8-16 words, Cambria, 13.5) words plus a running title (20-42 characters, Times or Cambria, 12). Abbreviations should be avoided.
Authors
Below the title, list all the authors with their complete affiliations. Each listed author must have an affiliation, which comprises the department, university, or organization and its location, city, state/province (if applicable), and country.
Place the e-mail address of the corresponding author at the bottom of the first page.
Abstract
A concise and factual unstructured abstract is required (150- 250 words). The abstract should state briefly the purpose of the research, the principal results, and major conclusions.
Keywords
4-6 keywords relevant to the article should be listed below the abstract.
Introduction
State the objectives of the work and provide an adequate background, avoiding a detailed literature survey or a summary of the results.
Material and methods
Provide sufficient detail to allow the work to be reproduced. Methods already published should be indicated by a reference: only relevant modifications should be described.
Results
Results should be clear and concise.
Discussion
This should explore the significance of the results of the work, not repeat them. A combined Results and Discussion section is often appropriate. Avoid extensive citations and discussion of
published literature.
Conclusions
The main conclusions of the study may be presented in a short conclusions section, which may stand alone or form a subsection of a Discussion or Results and Discussion section.
Acknowledgments
Contributions from anyone who does not meet the criteria for authorship should be listed, with permission from the contributor, in an Acknowledgments section.
Conflict of interest
Any interest, financial relationship, personal relationship, or religious or political beliefs that might influence the objectivity of the author can be considered as a potential source of conflict of interest. All manuscripts submitted to the journal must include a conflict of interest disclosure statement or a declaration by the authors that they do not have any conflicts of interest.
Financial support
Authors should list all funding sources and they are responsible for the accuracy of their funder designation.
Ethics statement
Studies involving humans and animals must have been performed with the approval of an appropriate ethics committee and provide the reference number.
Math formulae
Please submit math equations as editable text and not as images and numbers consecutively any equations that have to be displayed separately from the text.
e.g.
|
|
(2) |
Tables/Figures
Mention all the tables and figures in the text as follows:
(Table 1), (Figure 1)
References
The references should be listed on a separate sheet and should be numbered consecutively in the order in which they are cited in the text. Cite in the text by the appropriate Arabic number e.g. 1, 2, 3, and the numbers should be in the brackets. e.g [1], [1,3], [5-8], [5,6,12]
Reference list
Print articles
|
Article with 1 to 6 authors |
Author AA, Author BB, Author CC, Author DD. Title of article. Abbreviated title of the journal. Date of publication YYYY Mon DD; volume number (issue number): page numbers.
e.g. Petitti DB, Crooks VC, Buckwalter JG, Chiu V. Blood pressure levels before dementia. Arch Neurol. 2005 Jan;62(1):112-6. |
|
Article with more than 6 authors |
Author AA, Author BB, Author CC, Author DD, Author EE, Author FF, et al. Title of article. Abbreviated title of the journal. Date of publication YYYY Mon DD; volume number(issue number): page numbers.
e.g. Hallal AH, Amortegui JD, Jeroukhimov IM, Casillas J, Schulman CI, Manning RJ, et al. Magnetic resonance cholangiopancreatography accurately detects common bile duct stones in resolving gallstone pancreatitis. J Am Coll Surg. 2005 Jun;200(6):869-75. |
Electronic journal articles
|
Electronic journal article |
Author AA, Author BB. Title of article. Abbreviated title of Journal [Internet]. Date of publication YYYY MM [cited YYYY Mon DD]; volume number(issue number): page numbers. Available from: URL
e.g. Stockhausen L, Turale S. An explorative study of Australian nursing scholars and contemporary scholarship. J Nurs Scholarsh [Internet]. 2011 Mar [cited 2013 Feb 19];43(1):89-96. Available from: http://search.proquest.com/docview/ 858241255 |
|
Electronic journal article with DOI |
Author AA, Author BB, Author CC, Author DD, Author EE, Author FF. Title of article. Abbreviated title of Journal [Internet]. Year of publication [cited YYYY Mon DD]; volume number(issue number): page numbers. Available from: URL DOI
e.g. Kanneganti P, Harris JD, Brophy RH, Carey JL, Lattermann C, Flanigan DC. The effect of smoking on ligament and cartilage surgery in the knee: a systematic review. Am J Sports Med [Internet]. 2012 Dec [cited 2013 Feb 19];40(12):2872-8. Available from: http://ajs.sagepub.com/content/40/12/2872 DOI: 10.1177/0363546512458223 |
Conference articles
|
Print Conference |
Author(s). Title of paper. In: Editor A, Editor B, Editors. Title of Published Proceedings: Proceedings of the Title of Conference: subtitle of Conference; Year Month Date of Conference; Location of Conference. Place of publication: Publisher; Year of Publication. p. inclusive page numbers.
e.g. Luca J, Tarricone P. Does emotional intelligence affect successful teamwork? In: Kennedy G, Keppell M, McNaught C, et al, eds. Meeting at the Crossroads: Proceedings of the 18th Annual Conference of the Australasian Society for Computers in Learning in Tertiary Education, 2001 Dec 9-12; Melbourne: Biomedical Multimedia Unit, The University of Melbourne; 2001. p.367-76. |
|
Online Conference |
Author(s). Title of paper. In: Proceedings of the Title of Conference: subtitle of Conference [conference proceedings on the Internet]; Year Month Date; Location. Place of publication: Publisher; Year. Available from: Database Name. e.g. Cloherty SL, Dokos S, Lovell NH. Qualitative support for the gradient model of cardiac pacemaker heterogeneity. In: Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27 Annual Conference [conference proceedings on the Internet]; 2005 Sep 1-4; Shanghai, China. New York: IEEE; 2005 [cited 2013 Sep 2]. p. 133-6. Available from: IEEE Xplore |
Books and book chapters
|
Book : Print book OR Electronic book |
Author AA. Title of book. # edition [if not first]. Place of Publication: Publisher; Year of publication. Pagination.
e.g. Carlson BM. Human embryology and developmental biology. 4th ed. St. Louis: Mosby; 2009. 541 p.
Author AA. Title of web page [Internet]. Place of Publication: Sponsor of Website/Publisher; Year Published [cited YYYY Mon DD]. Number of pages. Available from: URL DOI: (if available)
e.g. Shreeve DF. Reactive attachment disorder: a case-based approach [Internet]. New York: Springer; 2012 [cited 2012 Nov 2]. 85 p. Available from: http://dx.doi.org/10.1007/978-1-4614-1647-0 |
Government and other reports
|
Government reports |
Author AA, Author BB. Title of report. Place of publication: Publisher; Date of publication. Total number of pages. Report No.: e.g. Rowe IL, Carson NE. Medical manpower in Victoria. East Bentleigh (AU): Monash University, Department of Community Practice; 1981. 35 p. Report No.: 4. |
|
Patent |
Name(s) of inventor(s). Assignee. patent title. Country or region of patent. Patent number. Date of patent. e.g. Clarke J, Pines A, McDermott RF, Trabesinger AH, inventors. University of California, assignee. SQUID detected NMR and MRI at ultralow fields. European Patent 1474707. 2004-11-10. |
Dictionaries and encyclopedias
|
Article from online reference work |
Title of encyclopedia [Internet]. Place of publication: Publisher; year. Title of article; [updated YYYY Mon DD; cited YYYY Mon DD]; [# of pages/screens]. Available from: URL
e.g. A.D.A.M. medical encyclopedia [Internet]. Atlanta (GA): A.D.A.M., Inc.; c2005. Ear barotrauma; [updated 2006 Oct 20; cited 2006 Nov 16]; [about 4 screens]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/001064.htm |
|
Article from electronic drug guide |
Title of work [Internet]. Place of publication: Publisher/Website; year. Name of the drug: [revision/review date; cited YYY Mon DD]; [# of pages/screens]. Available from: URL
e.g. AHFS consumer medication information [Internet]. Bethesda (MD): American Society of Health-System Pharmacists, Inc.; ©2008. Protriptyline; [revised 2007 Aug 1; reviewed 2007 Aug 1; cited 2008 Oct 2]; [about 5 p.]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a604025.html |
From the Internet
|
Web page: homepage part of website |
Author/organization’s name. Title of the page [Internet]. Place of publication: Publisher's name; Date or year of publication [updated yr month day; cited yr month day]. Available from: URL
e.g. Diabetes Australia. Diabetes globally [Internet]. Canberra ACT: Diabetes Australia; 2012 [updated 2012 June 15; cited 2012 Nov 5]. Available from: http://www.diabetesaustralia.com.au/en/ Understanding-Diabetes/Diabetes-Globally/
Title of the homepage [Internet]. Place of publication: Publisher's name; Date or year of publication. Title of specific page/part; Date of publication of part [Date cited of part]; [location or pagination of part]. Available from: URL
e.g. Australian Medical Association [Internet]. Barton ACT: AMA; c1995-2012. Junior doctors and medical students call for urgent solution to medical training crisis; 2012 Oct 22 [cited 2012 Nov 5]; [about 3 screens]. Available from: https://ama.com.au/media/junior-doctors -and-medical-students-call-urgentsolution-medical-training-crisis |
|
Image from web |
Note: If the title of the image is not shown construct a title that describes the image shown. Use enough words to make the constructed title meaningful. Place the constructed title in square brackets. Author or organization. Title [Image on the Internet]. Place of publication: Publisher's name; date of publication [date cited]. Available from: URL
e.g. Centers for Disease Control and Prevention. Shingles on the face [Image on the Internet]. 2011 [updated 2011 Jan 10; cited 2012 Nov 6]. Available from: http://www.cdc.gov/shingles/about/photos.html |
Manuscripts Submission Steps
Logging in/creating an author account, uploading and submitting the manuscript and supplementary files, and checking the status of your article (Submission page).
Decisions
Corresponding authors will be notified by a notification in their submission panel. Once a decision regarding their articles has been made. If any further actions are required, they will be outlined in the submission dashboard.
Revisions
When revision of a manuscript is requested, it is important that the authors carefully follow the instructions given in the Editor’s comments, which include reviewers’ comments point-by-point. The editor may request the author/s to revise a manuscript more than once
Proof-Reading
Before publication of manuscripts, authors must proofread their articles for correctness. It is the author’s responsibility to complete the proofreading within 7 days.
Copyright and Access
Bulletin of Pioneering Researches of Medical and Clinical Science is an Open Access journal, and all articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 (CC BY-NC-SA 4.0).

Archiving:
Remember that Creative Commons licenses aim to strike a balance between openness and respecting creators’ rights. Always review the full license for legal details.
Proofs and Reprints
Electronic proofs will be sent (In the corresponding author’s profile) to the corresponding author as a PDF file. Page proofs are considered to be the final version of the manuscript. Except for typographical or minor clerical errors, no major changes will be made to the manuscript at the proof stage. Because Bulletin of Pioneering Researches of Medical and Clinical Science will be published freely online, authors will have free electronic access to the full text (in both HTML and PDF) of the article.
Plagiarism
Permission from the copyright owner(s) of reproduced Tables, Figures, and Illustrations must be obtained, and a copy of the permission letter submitted to the Bulletin of Pioneering Researches of Medical and Clinical Science. The source must be acknowledged under the Table, Figure, or Illustration. The use of copied text, photographs, tables, or graphics from any source as one’s own is considered plagiarism, especially when a reference to the copied portion is not given.
Bulletin of Pioneering Researches of Medical and Clinical Science does not require all authors to sign the letter of submission. The corresponding author is responsible for ensuring that all authors have agreed to be listed and approved for the paper submission to the journal, and approved papers managing all communication between the journal and all co-authors, before and after publication. All manuscripts are checked for plagiarism using the iThenticate tool by Journal of Bulletin of Pioneering Researches of Medical and Clinical Science. Authors should strictly avoid plagiarism, including self-plagiarism. Manuscripts with over 15% overlapping from the similarity report results exceeding 15% would not be considered for publication by the Bulletin of Pioneering Researches of Medical and Clinical Science. Papers submitted to the Bulletin of Pioneering Researches of Medical and Clinical Science must be original and not be published or submitted for publication elsewhere.
The editorial board will thoroughly check submitted manuscripts to identify and prevent possible research misconduct regarding the publication of papers with Bulletin of Pioneering Researches of Medical and Clinical Science. If research misconduct is identified, according to the Editor’s and reviewers’ feedback, the corresponding author is responsible for retracting or correcting articles.
Withdrawal Policy
Once a manuscript has been accepted for publication in the journal, withdrawal is not permitted. However, if the withdrawal is eventually granted, the authors must pay the submission and processing fees, and submit to the Editorial Office, a document signed by all authors. Withdrawal of a published article is not an option, although such an article can be retracted for fraudulent reason/s.
Open access policy
Bulletin of Pioneering Researches of Medical and Clinical Science releases all academic papers under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 (CC BY-NC-SA 4.0) license. As an open-access journal, all content is freely available to users and their institutions without any fees.
For more details about the license, please visit: Creative Commons License Details.
Generative AI Usage Policy
With the remarkable advancements in Generative Artificial Intelligence (GenAI), academic publishing is entering a new era filled with both promising opportunities and complex challenges. To preserve transparency, authenticity, and scientific rigor, our journal has established the following policies regarding the use of GenAI technologies throughout the manuscript preparation, peer review, and publication processes:
Our journal embraces the potential of Generative AI to enhance scholarly communication, while steadfastly upholding the highest standards of ethical conduct and academic excellence. By implementing these policies, we strive to foster a culture of openness and integrity as we navigate this transformative frontier in scientific publishing.
Article Processing Charge
There is no fee for manuscript submission to Bulletin of Pioneering Researches of Medical and Clinical Science. However, Bulletin of Pioneering Researches of Medical and Clinical Science charges an article processing fee to the author(s). Because, our journal normally works by subscription, for the articles to have open access, the author(s) is/are required to pay an article processing charge. Bulletin of Pioneering Researches of Medical and Clinical Science is an open-access journal, meaning all articles are available online immediately upon publication to anyone, anywhere, at any time. To support open access, the journal has an Article Publishing Charge (APC). The author(s) will be asked to pay the Article Processing Charge (APC) [USD 150] to cover publications costs such as assigning DOI number, preparing the galley proof of the paper, professional editing. This is because the journal editors do not want rigorous work to be prevented from publication.
To help support researchers in low-to-middle-income countries, the Bulletin of Pioneering Researches of Medical and Clinical Science provides full and partial waivers of article processing charges for manuscripts based on the corresponding author's listed affiliation. Authors need to request these waivers in the Submission Process.
Our waiver policy follows a two-tier approach:
Waiver requests outside of these two tiers may not be considered.
Until preliminarily reviewed participation is voluntary so there is no penalty for refusing to participate, and the participants may withdraw at this time without penalty. After preliminary review, the author is not allowed to withdraw submitted manuscripts because the withdrawal is a waste of valuable resources that editors and referees spent a great deal of time processing submitted manuscripts, money, and works invested by the publisher. If the author still requests withdrawal of his/her manuscript when the manuscript is still in the peer-reviewing process, the author will be punished with paying APC per manuscript, as a withdrawal penalty to the publisher.
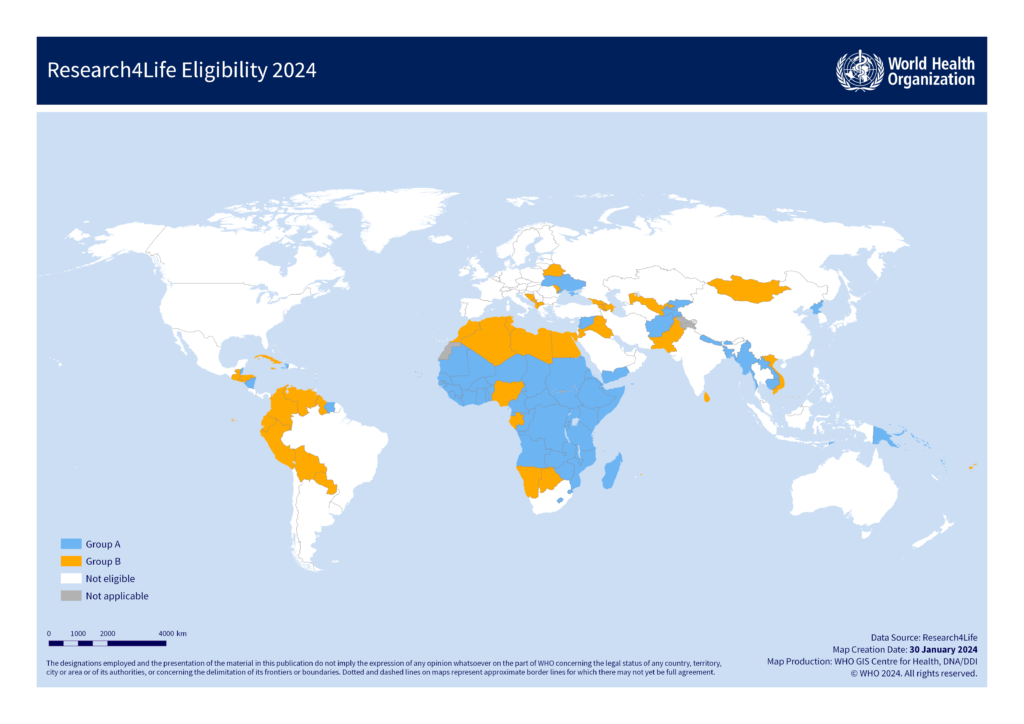
Countries, areas, and territories
Core Offer countries, areas, and territories
Group A (Free access)
|
|
Group B (Low-cost access)
|
|
Publishing Schedule
Bulletin of Pioneering Researches of Medical and Clinical Science is published online 2 times per year (Sep-Dec).
Announcement and Advertisement
Announcements regarding scientific activities such as conferences, symposium, are published for free. Advertisements can be either published or placed on website as banners.