The purpose of this research is to ascertain the prevalence of vaginal infections among females in Delta State across various age groups. Samples from female patients at Delta State's Central Hospitals and from women who visited private labs in Sapele, Abraka, Eku, and Oghara were used in the research. In order to detect vaginal infection, urine and higher vaginal swabs (HVS) were collected from 500 female subjects who had clinical signs of vaginitis. 88% of women had infected vaginal illnesses. A total of six (6) organisms were identified, namely; Staphylococcus spp., (54%; n=270), Pseudomonas aeruginosa (18%; n=90), Streptococcus sp., (16%; n=80), Klebsiella spp. (14%; n=70), Candida sp. (12%; n=60), and Escherichia coli (6%; n=30). 12% of the samples were not infected. Of the 500 samples examined, 120 (24%) samples had mixed infections, while 76% (n = 380) had a single infection. Age-wise distribution of the prevalence of vaginal infection reveals that a total of 90 samples were within the age group; of 14–19, with 66.7% appearing positive, 300 samples were within the age group of 20–30 years, with 96.76% (290) being positive, 110 samples were above the age of 30 years, with 81.8% (90) being positive. In this research, vaginal infections were very common. The age range of 20 to 30 years has a higher incidence of vaginal infections.
Introduction
The most prevalent health issue affecting women is vaginal infection, which has been related to a wide range of grave health risks. It is referred to as vaginitis in medicine and is a prevalent gynecologic disorder that accounts for 10 million doctor visits annually in Nigeria [1]. Normal mucous discharge from the female vagina keeps the vagina clean and moist. This common secretion is clear, odourless, and doesn't irritate. The existence of vaginal infections, or vaginitis, an inflammation or infection of the vagina, is suggested by a deviation from this norm [2]. According to reports, one of the most prevalent gynecologic conditions for which women seek treatment is vaginal infection (vaginitis). In addition to having serious consequences like a greater risk for STDs, vaginitis can be a sign of possible sexual abuse [3]. Vaginitis prevalence has been linked to several risk variables, including age, sexual activity, and sexual behavior. Studies have shown that vaginitis has a detrimental impact on women's quality of life, with some voicing anxiety, shame, and hygiene concerns, especially in those who experience recurrent symptoms [4].
The purpose of this research is to ascertain the prevalence of vaginal infections among females in Delta State across various age groups.
Significance of the study
Management of vaginal infections is based on the presenting aetiological agent. Thus, this study will provide information on the various aetiological organism responsible for vaginal infections which will aid in early recognition and treatment of the infection.
Background of the study
One of the most frequent gynaecological issues seen in both general medicine and gynaecological practise is vaginal infection [5]. The annual number of office appointments attributable to vaginitis ranges between 5 and 10 million [6]. Any irritation or infection of the vagina is referred to as vaginal infection, or vaginitis, in medical terminology. It affects women of all ages, with one-third of them experiencing vaginitis at some point in their lifetimes [7]. The muscular passageway between the uterus and the exterior genital region is known as the vagina. Vaginitis can happen when the vaginal walls swell up as a result of an irritant upsetting the delicate balance of the vaginal region [6]
Normal mucous discharge from the female vagina keeps the vagina clean and moist. This common secretion is clear, odourless, and doesn't irritate. A deviation from this pattern, however, denotes vaginitis, an illness or inflammation of the vagina [2]. Alterations of the normal vaginal condition are favoured by various factors including poor genital-anal hygiene, new or multiple sexual partners (regardless of the frequency of sexual intercourse), bathing in swimming pools or bathtubs, pregnancy, diabetes, parasitosis, urinary or faecal incontinence, stress, congenital malformations of the genital tract, frequent use of antibiotics, hormones, use of oral topical contraceptive preparations, vaginal medications, immunological deficiency, wearing tight clothing, smoking, presence of herpes simplex virus 2 (HSV-2) antibodies, and changes in the normal microbial flora such as a loss of production of H2O2 by lactobacilli [8].
According to Sim et al. and Ranjit et al. vaginal symptoms like discharge, odour, itching, irritation, or a burning feeling, as well as discomfort, are the most common complaints among patients who visit obstetrics and gynaecology clinics [9, 10]. However, some vaginal infections have few or no signs at all [11]. According to Mondeja et al. 20–62% of women of reproductive age experience vaginal infections, and about 20% of these cases are the outcome of changes brought on by birth control pills or antibiotics [11]. According to Martínez et al. between 24% and 37% of VIs are sexually transmitted, and 21.5 to 54.4% of them impact pregnant women [8].
Vaginal inflammation, or vaginitis, is brought on by a variety of infectious and non-infectious causes [12]. Vaginitis has been classified based on the cause, with the three most prevalent kinds being: Candida or "yeast" infection, bacterial vaginosis, and Trichomoniasis vaginitis [7]. A common health issue for women is vaginal disease, including bacterial vaginosis, candidiasis, and trichomoniasis [11]. There were many different types of pathogenic organisms found in the vaginal microflora. The preponderance of vaginal infections in women of reproductive age is caused by bacterial vaginosis, candidiasis, and trichomoniasis, respectively [13]. The findings of various studies conducted to determine how frequently the most prevalent infectious agents for vaginitis occur have varied. Between 8% and 75% of cases of BV, 2.2% to 30% of cases of VVC, and 0% to 34% of cases of trichomoniasis were found to be prevalent [7].
Materials and Methods
Study area
The study was undertaken using samples collected from females visiting the Central Hospitals and private laboratories spread across Sapele, Abraka, Eku, and Oghara, Delta State.
Collection of samples
A sample of 500 female individuals who had vaginal symptoms was taken. 500 pee and higher vaginal swabs (HVS) samples were collected from patients following the instructions in Cheesbrough [14] (for the HVS samples).
HVS samples and urine samples culture
The Nutrient, sabouraud dextrose, and blood agar were prepared and sterilized in an autoclave (Clifton, England) at 121℃ for 15 minutes. Thereafter the media were poured aseptically into plates after cooling to 45 ℃. Following collection, the urine and HVS samples were cultured on agar plates using the streak plate technique and incubated for 24 hours at 37 ℃.
Identification of microorganisms
The organisms obtained after using the streak plate method were then isolated in pure culture by picking a colony of each organism and inoculating it using a flamed wire loop on nutrient agar slants. These slants then served as a reservoir of the organisms obtained from each sample and were used for identification using the standard identification procedures of Cultural characteristics, Gram stain reaction, and biochemical methods such as catalase, coagulase, indole, sugar fermentation test, Simmons citrate, and hydrogen sulfide test.
Cultural characteristics (morphology)
Growth on solid media (plate culture)
The pattern of growth of the organisms on plate cultures was examined to determine the similarity to those recorded in approved literature.
Growth in differential media
The growth pattern and colour of the colonies on McConkey agar were observed to ascertain whether the collected organisms were lactose fermenters or not. It was specifically used to identify and confirm the presence of Escherichia coli.
In addition, the growth pattern and colour of the colonies on Mannitol agar were observed. This was done specifically to differentiate Staphylococcus aureus, a mannitol fermenter from Staphylococcus epidermis which does not ferment mannitol.
The growth pattern and colour of colonies on blood agar were also observed to identify Streptococci spp. [15].
Staining reactions
The clinical isolates were stained using the Gram Staining Technique developed by Christian Gram in 1884. A clean slide was wiped with cotton wool containing methylated spirit to remove grease. Thereafter, a flame-sterilized bacteriological wire loop was used to touch the top of a colony which was then emulsified with sterilized distilled water on the slide until a smooth homogenous suspension was obtained. The slide was allowed to dry completely in air and the organisms fixed to the slide by passing it twice over a Bunsen flame and placed to cool on a staining rack over a sink. The organisms were first flooded with crystal violet (BDH, England) for 30 seconds and washed off with slow-flowing water. The film was then treated with Lugol iodine (Fischer Company, USA) to fix the stain on the cell for 1 minute. The slide was washed with alcohol and counter-stained with saffranine for 2 minutes. The secondary dye was washed off slowly with water and the slide was allowed to dry in air before examination. The slides were mounted and examined under the microscope, first under a low-power objective (to select a suitable field) and then under the oil immersion objective.
Biochemical reactions
Indole test
1.5 g of peptone water was dissolved in 100 of water and autoclaved at 120 ℃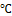 for 15 minutes. 5 ml of the medium was transferred using a pipette into sterilized bottles, inoculated with the test organisms, and incubated for up to 24 hours. After incubation, the culture broth was then added with 3–5 drops of Kovac's reagent (isoamyl alcohol, para dimethylaminobenzaldehyde, and concentrated hydrochloric acid), and the existence or absence of a red ring at the reagent layer of the medium's surface was checked. A red ring signifies a positive test when it's present.
for 15 minutes. 5 ml of the medium was transferred using a pipette into sterilized bottles, inoculated with the test organisms, and incubated for up to 24 hours. After incubation, the culture broth was then added with 3–5 drops of Kovac's reagent (isoamyl alcohol, para dimethylaminobenzaldehyde, and concentrated hydrochloric acid), and the existence or absence of a red ring at the reagent layer of the medium's surface was checked. A red ring signifies a positive test when it's present.
Hydrogen sulphide test
Lead acetate paper strips are used for the detection of hydrogen sulfide production by microorganisms. 1.5 g of peptone water was dissolved in 100 ml of water and autoclaved at 121 ℃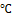 for 15 minutes. 5 ml of the medium was transferred using a pipette into sterilized bottles and inoculated with the test organisms. Lead acetate paper strips were then immersed between the plug and the inner wall of the bottle, covered, and incubated at 35-37 ℃
for 15 minutes. 5 ml of the medium was transferred using a pipette into sterilized bottles and inoculated with the test organisms. Lead acetate paper strips were then immersed between the plug and the inner wall of the bottle, covered, and incubated at 35-37 ℃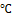 for 24 hours. The blackening of the paper strips indicates a positive test.
for 24 hours. The blackening of the paper strips indicates a positive test.
Simmons citrate test
The Simmons citrate agar was prepared by dissolving 2.43 g in 100 ml of water and autoclaved at 121℃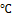 for 15 minutes. It was cooled to 45 ℃
for 15 minutes. It was cooled to 45 ℃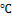 , poured on plates, and allowed to solidify. After which the organisms to be tested were then inoculated on the plates by streaking using flame sterilized wire loop and incubated for 24 hours. A blue colouration indicates a positive test. As described by Oghenemaro et al. [15].
, poured on plates, and allowed to solidify. After which the organisms to be tested were then inoculated on the plates by streaking using flame sterilized wire loop and incubated for 24 hours. A blue colouration indicates a positive test. As described by Oghenemaro et al. [15].
Results and Discussion
|
Table 1. Prevalence of Organisms Diagnosed from Urine and HVS Samples |
||
|
Infective Organism |
No. of females examined |
No. of positive samples |
|
Staphylococcus spp. |
500 |
270(54%) |
|
Streptococcus spp. |
500 |
80(16%) |
|
Escherichia coli |
500 |
30(6%) |
|
Klebsiella spp. |
500 |
70(14%) |
|
Pseudomonas aeruginosa |
500 |
90(18%) |
|
Candida sp. |
500 |
60(12%) |
|
None |
500 |
60(12%) |
Table 1 shows the prevalence of organisms diagnosed from urine and HVS samples of females. The prevalence of infected vaginal infection in this study was 88%. A total of six organisms were identified namely; Staphylococcus spp., Streptococcus spp., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, and Candida sp. 12% (n = 60) of the samples examined were devoid of infectious organisms, thus were not infected. The most prevalent organism identified was Staphylococcus spp., (54% n=270), which is closely followed by Pseudomonas aeruginosa (18%; n=90), streptococcus sp. (16%; n=80), Klebsiella spp. (14%; n=70), Candida sp. (12%; n=60). The least prevalent organism identified was Escherichia coli (6%; n=30).
|
Table 2. Mixed and Single Infections in Urine and HVS Samples |
||
|
No of the samples examined |
No with mixed infections |
No with single infections |
|
500 |
120 (24%) |
380 (76%) |
Table 2 shows the prevalence of mixed and single infections. Of the 500 samples examined, a total of 120 (24%) samples had mixed infections (more than one infectious organism), while 76% (n = 380), had a single infection.
|
Table 3. Prevalence of Infection about Age |
|||
|
Age |
No. of Female samples |
No. of Positives |
No. of Negatives |
|
14-19 |
90 |
60 (66.7%) |
30 (33.3%) |
|
20-30 |
300 |
290 (96.76%) |
10 (3.3%) |
|
Above 30 |
110 |
90 (81.8%) |
20 (18.2%) |
|
Total |
500 |
440(88%) |
60(12%) |
Table 3 shows the distribution of infection among ages. 90 female samples were within the age group; 14-19, with 66.7% appearing positive. 300 samples examined were within the age group of 20-30 years, however, 96.76% (290) were positive. A total of 110 samples examined were above 30 years of age, with 81.8% (90) being positive. The overall prevalence of vaginal infection was 88% (n = 440). The age group of 20-30 years were more represented in this study, and had the highest prevalence of vaginal infection, followed by those above 30 years, with the least being 14-19 years.
Vaginal infection is a widespread gynecological problem among females, especially reproductive females. This study aimed to determine the prevalence of vaginal infection among females and evaluate the age-wise distribution.
According to the study's results, females in the community under study had vaginal infections at a high prevalence of 80%. Al-Mamari who claimed a prevalence of 74%, made a similar finding [7]. A prevalence of 81% among female students at the University of Calabar was also found by Cummins Jr et al. [16]. By Bhargava et al. a lower prevalence of 46.96% was found. Both established and developing nations struggle with a serious public health issue related to vaginal infections [17]. The effects of females' high vaginitis prevalence have not gotten enough focus.
Vaginal infection is a widespread gynecological problem among females, especially reproductive females. This study aimed to determine the prevalence of vaginal infection among females and evaluate the age-wise distribution.
The results of this research have demonstrated that females in the community under study have vaginal infections, with a prevalence of 80%. Al-Mamari who reported a prevalence of 74%, found comparable findings [7]. Additionally, 81% of female students at the University of Calabar were affected, according to Cummins Jr et al. study [16]. Bhargava et al. found a lower prevalence of 46.96% [17]. Both in established and developing nations, vaginal infections are a significant public health issue. There hasn't been enough focus on the effects of the high incidence of vaginitis in women.
Six aetiological agents were found in this study's community, including Staphylococcus species, Streptococcus species, Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, and Candida species. Gardnerella vaginalis, Lactobacillus species, Streptococcus agalactiae, Candida albicans, C. tropicalis, and C. glabrata are some of the organisms that Al-Mamari discovered [7].
Six aetiological agents were found in this study's community, including Staphylococcus species, Streptococcus species, Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, and Candida species. Organisms isolated by Al-Mamari include Gardnerella vaginalis, Lactobacillus spp., Streptococcus agalactiae, Candida albicans, C. tropicalis, and C. glabrata [7]. In this current study, the most prevalent organism identified was Staphylococcus spp. (54%; n = 270), which is closely followed by Pseudomonas aeruginosa (18%; n = 90), Streptococcus sp., (16%; n = 80), Klebsiella spp. (14%; n = 70), and Candida sp. (12%; n = 60). The least prevalent organism identified was Escherichia coli (6%; n = 30). Of the organisms, the majority were bacteria, while one was yeast. Thus, bacterial vaginosis and vulvovaginal candidiasis were responsible for vaginal infection, with bacterial vaginosis being the most prevalent. This is similar to the findings of Martínez et al. who reported vaginal infections due to bacteria as the most common cause. Bhargava et al. also reported that the majority of those with vaginal infections had bacterial vaginosis [17]. Al-Mamari stated that BV was the second most common infection, coming in at 43 (29%) and that VVC was the most common, with 68 (49%). According to Cummins Jr et al. 180 people (35.29%) had bacterial vaginosis, whereas 330 (64.71%) had candidiasis [16]. No trichomoniasis cases were found in the current research. Al-Mamari provided a comparable summary [7]. Alli et al. did find a 2% incidence of trichomoniasis [18].
Pathogens resulting in vaginal infections may occur as single infections or as co-infections (mixed infections). In this study, a total of 120 (24%) samples had mixed infections (more than one infectious organism), while 76% (n = 380) had a single infection. Atting et al. also reported the presence of co-infection in the pathogenesis of vaginal infections [1]. Cummins Jr et al. reported a prevalence of 51% single infections and 19% mixed infections [16]. Bhargava et al. reported the prevalence of mixed infection to be 18.1% [17].
In this present study, age-wise distribution of the prevalence of vaginal infection was carried out. A total of 90 samples were within the age group 14–19, with 66.7% appearing positive. A total of 300 samples examined were within the age group of 20-30 years, however, 96.76% (290) were positive. A total of 110 samples examined were above 30 years of age, with 81.8% (90) being positive. 88% of women (n = 440) had vaginal infections overall. The age range from 20 to 30 years had the greatest prevalence of vaginal infection in this research, followed by those over 30 years, with 14 to 19 years having the lowest prevalence. Similar findings were earlier reported by Al-Mamari who found that married women between the ages of 21 and 35 had a significantly higher incidence of vaginal infections [7]. The outcome was comparable to that reported by Gibney et al. in Nigeria, who discovered that women between the ages of 26 and 30 had the highest incidence of vaginal infection. According to Attinge et al. people between the ages of 16 and 30 had the greatest prevalence of infections. According to Cummins Jr et al. respondents between the ages of 21 and 25 had the greatest prevalence of the infection (76.36%) [16]. According to Bhargava et al. vaginitis was more prevalent in women aged 20–29 (56.1%) [17]. The greatest prevalence may be related to the age group of 21 to 35 years being the most reproductively active and having a high rate of sexual exposure. Oghenemaro et al. however, found the opposite results, stating that women between the ages of 36 and 45 were more likely to experience vaginal infections [15].
Conclusion
It has been investigated how common vaginal diseases are. This research found a high prevalence of vaginal infections. In this study, vaginal infections were more common in women aged 20 to 30. The majority of the samples examined were obtained from females in the age range of 20–30 years. This could infer that vaginal symptoms are more common in this age group.
Recommendations
Based on the findings of this study, the following recommendations are given:
Females should be educated on the risk factors associated with vaginal infections.
Further studies should assess factors that increase susceptibility to vaginal infections in the same population.
Acknowledgments: None.
Conflict of interest: None.
Financial support: It was funded by the Department of Pharmaceutical Microbiology and Biotechnology. Delta State University, Abraka.
Ethics statement: Ethical clearance was obtained from the Research and Ethics Committee, Faculty of Science, Delta State University, Abraka, Nigeria at the meeting held on 3rd October 2022. Ref: REC/FOS/DEL/22/03/10.